

One atom of carbon-12 has a mass of 12 atomic mass by definition. The Atomic Mass Data Center and its electronic bulletin are tools for the exchange of information on experimental masses, evaluation of masses, extrapolation of masses, and theoretical masses. The carbon-12 nuclide was chosen as the standard reference against which all additional masses were measured. While comparing masses measured in grams is useful, it is significantly more feasible to have a standard that allows us to evaluate comparative atomic masses more easily. The mass of an oxygen-16 atom, for example, is 2.66×10 23 g. atomic mass unit and discusses prospects of replacing the prototype kilogram by an atomic mass standard. It is possible to estimate such tiny masses using a contemporary technology known as a mass spectrometer. a unit of mass, equal to 112 the mass of the carbon-12 atom and used to express the mass of atomic and subatomic particles. SynonymsEdit dalton unified atomic mass unit. Individual atom masses are extremely tiny. (chemistry) A unit of mass equal to 1/12 the mass of an atom of the 12C isotope of carbon. One amu = The aggregate of the proton and neutron rest masses.ġ amu is equal to 1.67377 x 10 -24 grams or 1.67377 x 10 -27 kilograms.ġamu = (Mass of 1 126C atom divided by 12) U-238, the most common natural isotope of uranium, contains 3 more neutrons than U-235, which has been utilized in nuclear reactors and atomic bombs. For instance, uranium-235 (U-235) does have an AMU of roughly 235, whereas uranium-238 (U-238) has a marginally greater AMU.
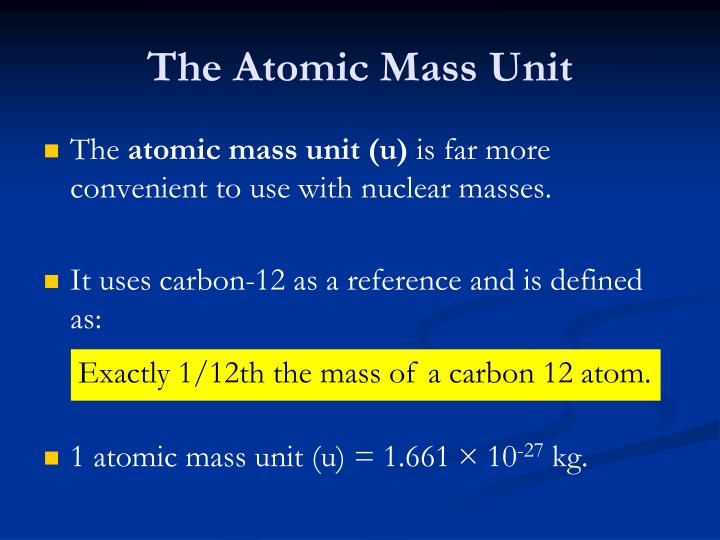
The AMU is used to represent and differentiate between distinct isotopes of elements by expressing their relative masses. In AMU, an atom’s mass is generally equal to the nucleus’ total amount of protons and neutrons. One AMU is the sum of the proton and neutron rest masses, in imprecise words.ġ.67377 x 10 -27 kilogramme (kg) or 1.67377 x 10 -24 gramme (g). Type the number of Atomic mass unit (u) you want to convert in the text box, to see the results in the table. It is defined to be 1/12 of the mass of one atom of carbon-12. The isotopic mass usually differs for other isotopes. The unified atomic mass unit (u), or dalton (Da), is a small unit of mass used to express atomic and molecular masses. For 12 C, the atomic mass is exactly 12u since the atomic mass unit is defined from it. A unit of measurement like 1 Atomic Mass Unit, Unified (u AMU) is a definite magnitude of a quantity, defined and adopted by convention or by law, that is used as a standard for measurement of the same quantity in Mass. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. The nucleus of the carbon-12 (C-12) atom includes 6 protons and 6 neutrons. The kilogram (SI unit symbol: kg) is the base unit of mass in the Metric system and is defined as being equal to the mass of the International Prototype of the Kilogram.more definition+ In relation to the base unit of mass weight > (kilograms), 1 Atomic Mass Unit (u) is equal to 1.660540199E-27 kilograms, while 1 Kilograms (kg) 1 kilograms. One atomic mass unit is equal: 1u 1.66 x 10 -24 grams. Answer: The atomic mass unit (referred to simply as AMU or amu) is described as 1/12 of the mass of a carbon-12 atom.


 0 kommentar(er)
0 kommentar(er)
